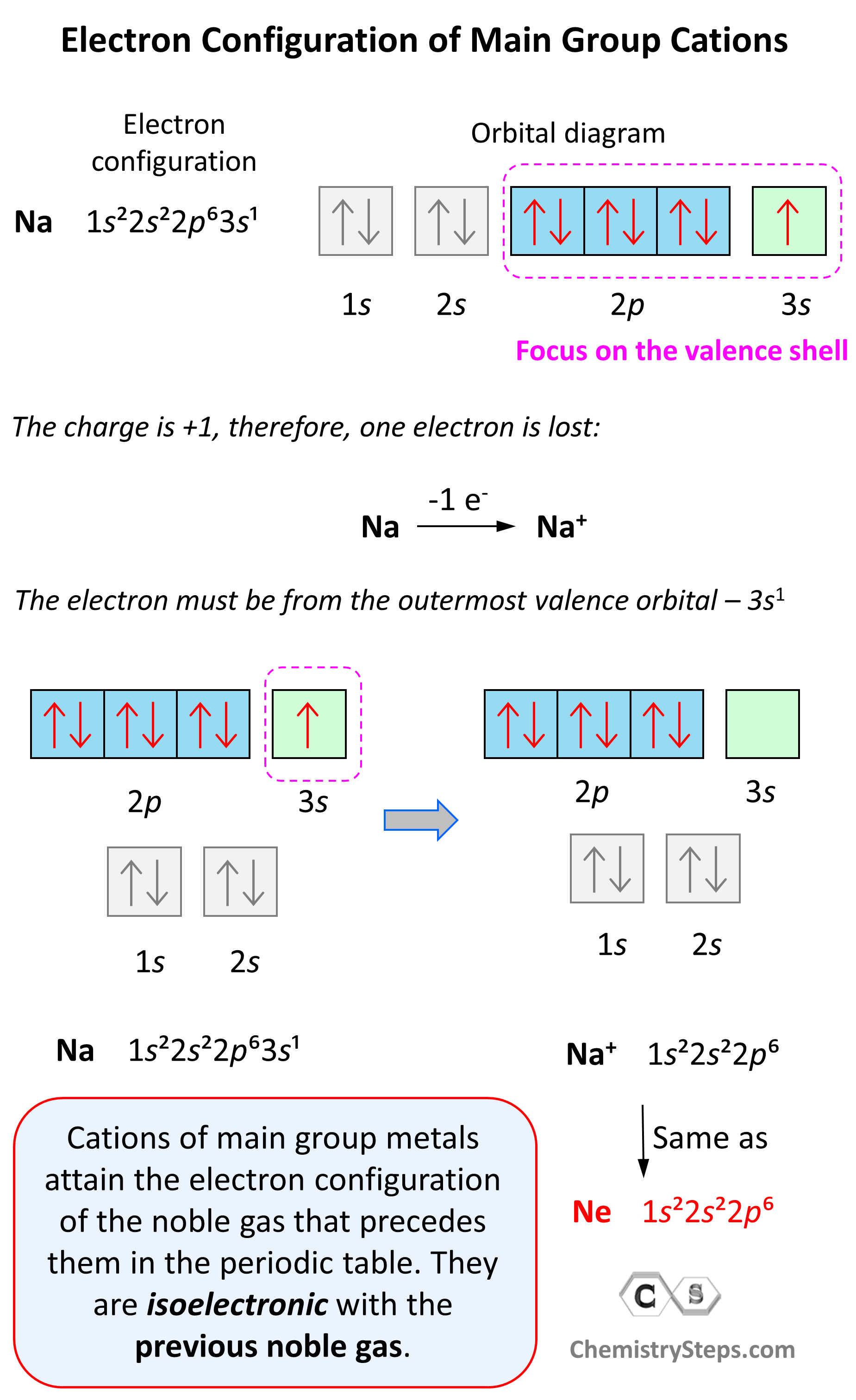
How Do Ionic Electrons Work . Web write the electron configuration of oxygen atom (z=8). Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; It starts with a simple picture. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. This page explains what ionic (electrovalent) bonding is. Web first, write the electron configuration for the neutral atoms: How many electrons must o lose/gain to achieve octet? Next, remove electrons from the. Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a.
from general.chemistrysteps.com
Next, remove electrons from the. This page explains what ionic (electrovalent) bonding is. It starts with a simple picture. Web first, write the electron configuration for the neutral atoms: Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Web write the electron configuration of oxygen atom (z=8). Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. How many electrons must o lose/gain to achieve octet?
Electron Configurations of Ions Chemistry Steps
How Do Ionic Electrons Work It starts with a simple picture. Next, remove electrons from the. This page explains what ionic (electrovalent) bonding is. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Web write the electron configuration of oxygen atom (z=8). How many electrons must o lose/gain to achieve octet? It starts with a simple picture. Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web first, write the electron configuration for the neutral atoms:
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica How Do Ionic Electrons Work Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. How many electrons must o lose/gain to achieve octet? Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Web since electrons are negatively charged, an atom that. How Do Ionic Electrons Work.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry How Do Ionic Electrons Work This page explains what ionic (electrovalent) bonding is. Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Web since electrons are negatively. How Do Ionic Electrons Work.
From www.coursehero.com
[Solved] Ionic Bonds Worksheet Part A Valence Electrons and Charges How Do Ionic Electrons Work Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. How many electrons must o lose/gain to achieve octet? It starts with a simple picture. Web first,. How Do Ionic Electrons Work.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise How Do Ionic Electrons Work Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. Web a cation. How Do Ionic Electrons Work.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds How Do Ionic Electrons Work Web first, write the electron configuration for the neutral atoms: Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Next, remove electrons from the. It starts with a simple picture.. How Do Ionic Electrons Work.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? How Do Ionic Electrons Work Next, remove electrons from the. It starts with a simple picture. Web write the electron configuration of oxygen atom (z=8). This page explains what ionic (electrovalent) bonding is. Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged. How Do Ionic Electrons Work.
From wou.edu
CH104 Chapter 3 Ions and Ionic Compounds Chemistry How Do Ionic Electrons Work Web first, write the electron configuration for the neutral atoms: Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Next, remove electrons from the. Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. Web ionic bond, type of. How Do Ionic Electrons Work.
From www.youtube.com
Valence Electrons & Ionic Charge and the Periodic Table YouTube How Do Ionic Electrons Work Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Web first, write the electron configuration for the neutral atoms: How many electrons must o lose/gain to achieve octet? Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in. How Do Ionic Electrons Work.
From www.showme.com
Showing electron transfer in ionic compounds Science ShowMe How Do Ionic Electrons Work Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Web write the electron configuration of oxygen atom (z=8). This page explains what ionic (electrovalent) bonding is. Web a cation (a positive ion) forms when. How Do Ionic Electrons Work.
From en.wikipedia.org
Ionic bonding Wikipedia How Do Ionic Electrons Work It starts with a simple picture. How many electrons must o lose/gain to achieve octet? This page explains what ionic (electrovalent) bonding is. Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Next, remove electrons from the. Web write the electron configuration of oxygen atom (z=8). Web ionic bond, type of. How Do Ionic Electrons Work.
From inovasibiologi.com
Ikatan Kimia INOVASI BIOLOGI How Do Ionic Electrons Work Web write the electron configuration of oxygen atom (z=8). Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. It starts with a simple picture. Next, remove electrons from the. Web first, write the electron configuration for the neutral atoms: How many electrons must o lose/gain to achieve. How Do Ionic Electrons Work.
From study.com
Factors Influencing the Formation of Ionic Bonds Lesson How Do Ionic Electrons Work Web first, write the electron configuration for the neutral atoms: This page explains what ionic (electrovalent) bonding is. Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Next, remove. How Do Ionic Electrons Work.
From www.youtube.com
Electron Configuration of Ions YouTube How Do Ionic Electrons Work Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; It starts with a simple picture. Web first, write the electron configuration for the neutral atoms: This page explains what ionic (electrovalent) bonding is. Next, remove electrons from the. Web ionic bond forms when the valence (outermost) electrons of one atom are. How Do Ionic Electrons Work.
From stock.adobe.com
Ionic covalent bonds examples. Chemical structural models. Atoms How Do Ionic Electrons Work It starts with a simple picture. Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Web first, write the electron configuration for the neutral atoms: Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web a cation (a positive ion) forms when a. How Do Ionic Electrons Work.
From sciencenotes.org
Ionic Bond Definition and Examples How Do Ionic Electrons Work It starts with a simple picture. How many electrons must o lose/gain to achieve octet? Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web first, write the electron configuration for the neutral atoms: Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a.. How Do Ionic Electrons Work.
From www.youtube.com
Ion Electron method Acidic and Basic Medium Chemistry ask YouTube How Do Ionic Electrons Work Next, remove electrons from the. Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; How many electrons must o lose/gain to achieve octet? Web ionic bond,. How Do Ionic Electrons Work.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation, free download ID3983384 How Do Ionic Electrons Work Web write the electron configuration of oxygen atom (z=8). Web a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. This page explains what ionic (electrovalent) bonding is. Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Web. How Do Ionic Electrons Work.
From www.slideshare.net
Ionic bondingChemistry How Do Ionic Electrons Work Web ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. It starts with a simple picture. How many electrons must o lose/gain to achieve octet? Web since electrons are negatively charged, an atom that loses one or more electrons will become positively charged; Web first, write the electron. How Do Ionic Electrons Work.